Operation - Secondary ions
Sputtering produces secondary ions that are used for SHRIMP analysis. Most of the material that is sputtered off the target are neutrals but there is a big difference between different elements as to what fraction is ionized. Alkali metals easily give up their electrons and their ion yields are very high as positive ions. Halogens are always looking for an electron and so their yields as negative ions are very high. The propensity for ionization therefore follows the periodic table. Some elements (Zn, Hg, N) have very poor atomic ion yields. For nitrogen analysis though, the yield of the negatively ionized CN molecule is very high allowing abundance and isotopic measurement in carbonaceous matrices.
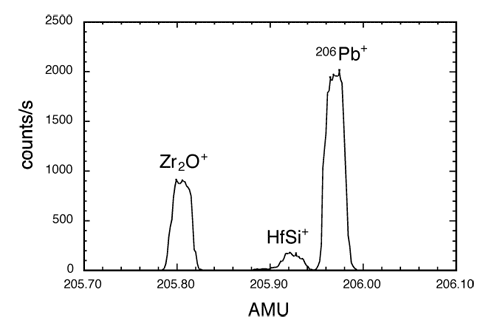
Mass spectrum of mass 206 from zircon showing the Pb-206 peak and other peaks associated with molecular fragments.
 menu
menu